Healthcare Professionals
Our Clinical Trials of SDT are planned for 2026. Currently, we are completing SwissEthics regulatory compliance whilst we complete our first funding round. Below is the information required to inform you and your patients about how and why we believe SDT to be the most exciting development in the field of GBM: safe, repeatable, precision medicine.
Our Mission: To make Sonodynamic Therapy (SDT) the Third Treatment for Brain Tumours, after surgery and Chemo/Radiotherapy.
Your Call to Action...
We are not yet recruiting but will update the website and contact relevant neuro-oncologists when we have the necessary permissions and processes in place to start. Watch this space.
Background
Glioblastoma (GBM) is the most frequent malignant primary brain tumor in adults. Despite standard of care (SOC) treatment with maximal resective safe surgery, radiotherapy and chemotherapy, median overall survival from diagnosis is only 14-15 months (or 6-8 months from tumour recurrence (recGBM) after SOC). There is an unmet need for safe and minimally invasive interventions that could treat GBM rapidly, when recurrence is noted in 3 monthly MRI surveillance scans.
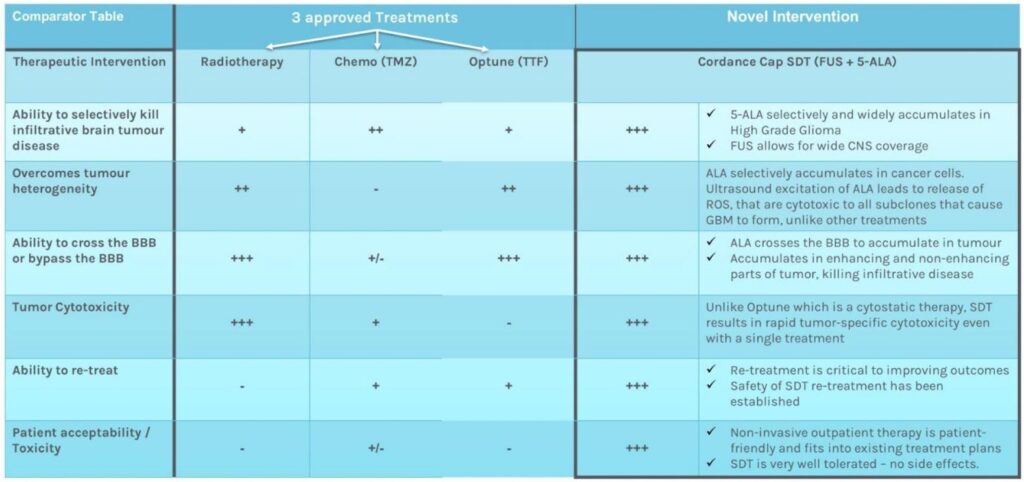
Sonodynamic therapy offers a means to combat the unique heterogeneity of GBM, as well as its diffuse infiltrative nature that makes it so difficult to treat. It involves ingestion of a ‘sonosenitiser’ drug (5-ALA) that leads to accumulation of its active metabolite, PPIX, in cancerous cells, not matter what their molecular subtype is – i.e. it will accumulate in cancer cells that are have survived and are resistant to radiotherapy and chemotherapy (with >95% sensitivity – no chemotherapeutic offers this degree of ‘precision medicine‘).
Delivering focused ultrasound energy promotes selective cell kill through generation of reactive oxygen species, whilst sparing surrounding normal brain tissue: sonodynamic therapy (SDT) (1). Early safety studies with other ultrasound-delivery devices have yielded incredibly promising results with a doubling of median survival to 15mths for patients with recGBM (4,11) and 19mths for DIPG (a paediatric brainstem tumour with median life expectancy of 9mths) – pers. comm. S. Marcus, SonALAsense USA.
Rationale
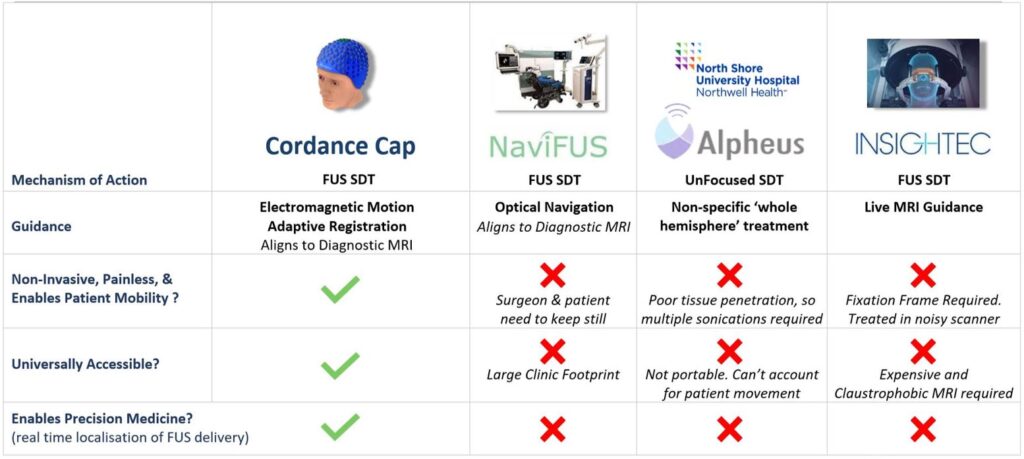
No novel agent has added more than 3-4 months life expectancy to people with GBM. The lack of local or systemic toxicity observed after trials of SDT in malignant brain tumours, and the lack of efficacy of current first line therapy for GBM, motivated us to develop a fully navigatable FUS device – the ‘Cordance Cap’ that uses over 400 220KHz ultrasound transducers to focus energy to any part of the brain (US Patent 18/146,028) and therefore focally treat recurrent GBM. The ‘Cordance Cap’ has received U.S. FDA Fast Track Designation for use in human liquid biopsy tests and has been shown to be safe in a successful human efficacy trial (2), with a further 40 patients to be recruited in a new trial of sonobiopsy: https://clinicaltrials.gov/study/NCT05281731. It was designed to open the blood brain barrier but, like the FDA-approved 220Khz commercially available Exablate Type 2.0 FUS device, it can also be used to activate ALA and non-invasively treat brain tumours via SDT (3,5,6).
The sensitivity of SDT to selectively kill cancer cells whilst sparing normal brain tissue may explain the lack of serious adverse events (SAEs) seen thus far in early clinical safety studies. Moreover, there is little treatment burden for the patient, as they are awake and without the need for general anaesthesia during their outpatient treatment. SDT will not lead to postponement of any second-line / salvage adjuvant therapy. As a substantial additional benefit, SDT works as a potential auto-immune vaccine via its abscopal effect. This hypothesis is based on clinical trials using laser light to activate ALA, which showed that not only was the targeted tumour destroyed by ALA activation but also satellite and distant lesions, suggesting that the tumour antigens released by photodynamic therapy (PDT) are recognised by the immune system, in the same way that vaccines also cause an immune response. We believe that SDT works by the same pathological processes as PDT*. The abscopal effect is discussed in more detail in a review by Collins et al. (9).

At the time of writing, 40+ adults and 15 children have already been treated with SDT for recGBM (e.g. 3,4,7,10i) and DIPG (5) respectively with no adverse events. The Cordance Cap uses broadly similar sonication parameters to the devices that have already been used to deliver SDT, so we are investigating its feasibility and safety to incorporate its use for patients with recGBM. Secondary end points of the trial are to assess radiological evidence of cell kill (with MRI scans performed the day after SDT treatment and monthly for three further months), progression free survival and overall survival.
Our study aims to prospectively investigate whether SDT can be integrated into the existing care pathway of glioblastoma patients undergoing routine MRI surveillance after completion of the SOC Stupp protocol (8).
Data obtained from our patient participation and inclusion (PPI) survey suggests that people with GBM would be willing to undertake an outpatient procedure such as SDT in addition to their 3 monthly MRI scans, especially as early results show a doubling in survival time for recGBM (4). Feedback was positive for SDT being a non-invasive outpatient procedure, using existing MRI scans and the Cordance cap offers the benefit of being cheap and portable enough to allow a large network of hospitals to provide SDT close to home, reducing travel time that is a considerable burden for patients and their family / carers – particularly if they have a neurological deficit. Moreover, SDT has been proved safe in children with DIPG (5), unlike many experimental chemotherapeutics which are often not even tested in children. Thus, SDT is being investigated for the whole population – including those deemed too medically unfit or too frail for chemotherapy, as the treatment is so gentle.
Risks
As no SAEs are yet to be reported with SDT, there is only a theoretical risk of seizures, intra-tumoural swelling and haemorrhage (with subsequent neurological deterioration in the first few hours after treatment). There are theoretical risks with the use of ALA too (allergic reaction, abnormal liver function and sunburn to exposed skin), However, all patients will have had their initial tumour debulking surgery with the use of 5-ALA as a fluorescent-guided tool (which means that we know the 5-ALA will accumulate in their recGBM and that they have not suffered side effects from it before). Patients will be monitored closely for side effects / SAEs and advised to wear SPF50 and avoid direct sunlight to exposed skin for 24hrs after taking ALA. However, asEven though these SAEs have never yet been seen, we have informed the Ethics board that the study objectively poses more than minimal but still justifiable and controlled risks.
Redo Surgery
If a patient requires redo surgery as SOC, they will not be excluded from the trial and SDT will be offered 1-2 days prior to their schedulaed operation. This has the added benefit of allowing us to collect spare biopsied tissue to look for histological markers of cell death, as an additional end point of the trial.
Sex assigned at birth and gender will be recorded and considered in the analysis, as biological differences may influence glioblastoma progression and treatment response. Inclusive language will be used in all participant-facing materials, and no exclusion will be made based on sex or gender.
Stage of Trial
We have completed a trial protocol (see flow chart below) and secured funding for the Cordance Cap purchase and its QA tests to allow use in patients with GBM. Ethical approval for a single site feasibility trial in Bern will be submitted in September 2025, with the aim of starting the trial in Q2 2026.
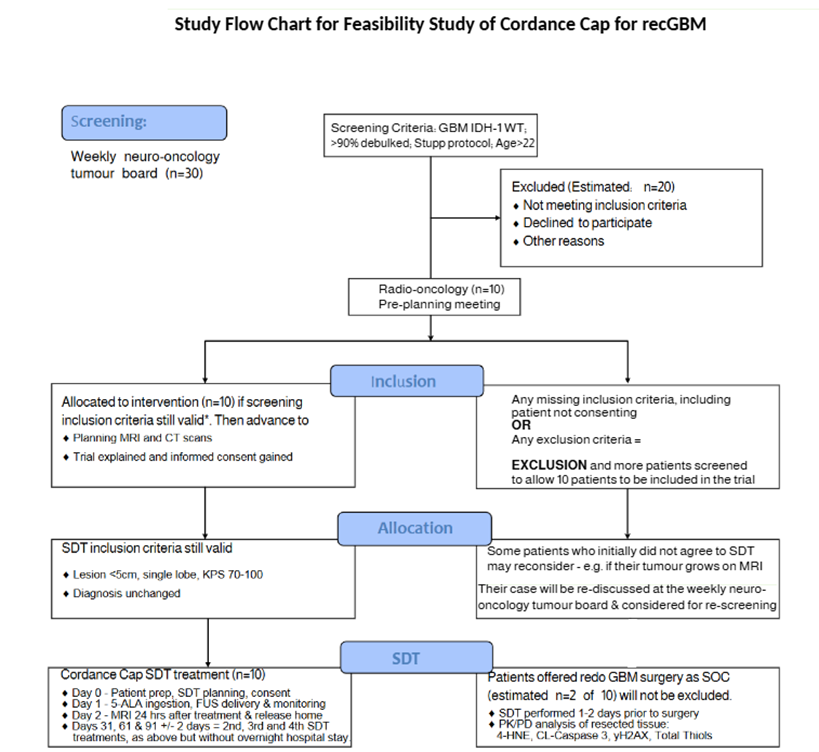
Study Inclusion/ Exclusion Criteria
- IDH-1WT rec GBM on Stupp protocl
- Age >22yo; KPS 70-100
- Able to consent
- Cumulative RT dose <64Gy
- No pregnancy or breastfeeding
- No coagulopathy or sig. liver deisease
- No dissemination or multifocal disease
- No allergy to 5-ALA or contrast agents
- No porphyrias, HIV or Long COVID
- No history of stroke or spontaneous intra-tumoural bleeding
- No significant peritumoural oedema requiring urgent intervention (surgical or medical)
- Not on another experimental trial drug
If you have a patient who you think would be eligible for the trial, please ask them to contact us at info@cureGBM.co.uk. Alternatively, if they are a Swiss resident who underwent SOC treatment in Switzerland, they can be referred directly to the PI, Prof Philippe Schucht at the department of neurosurgery, InselSpital, Bern, for discussion at the local tumour board meeting.
By evaluating feasibility and safety, this study will generate generalisable knowledge needed to inform the design of future randomised or controlled trials.
Information for Researchers
References: Existing Evidence for Safety and Efficacy of Sonodynamic Therapy for Malignant Brain Tumours
See annotated reference list below and tables further below comparing SDT to other approved treatments for GBM and comparing the Cordance Cap to the NaviUS, CV- 01 and Exablate FUS delivery devices.
SDT Webinar hosted by the Focused Ultrasound Foundation, September 28 2023: https://www.fusfoundation.org/posts/focused-ultrasound-and-sonodynamic-therapy-webinar/
- A review / explanation of how 5-ALA is activated by Focused Ultrasound that results in the selective cell kill seen in Sonodynamic Therapy (SDT). It includes the preclinical research that showed that 3 (weekly) SDT treatments in a rat model of glioma caused complete remission / cure:
Marcus SL & de Souza MP. Theranostic Uses of the Heme Pathway in Neuro-Oncology: Protoporphyrin IX (PpIX) and Its Journey from Photodynamic Therapy (PDT) through Photodynamic Diagnosis (PDD) to Sonodynamic Therapy (SDT). Cancers (Basel). 2024 Feb 10;16(4):740.
- Research paper demonstrating that the Cordance Cap prototype FUS delivery device can be used safely in patients with GBM (note the cap was used to successfully open the blood brain barrier in this study of 5 GBM patients). Our study will be the first to use the Cordance Cap for SDT:
Yuan, J., Xu, L., Chien, CY. et al. First-in-human prospective trial of sonobiopsy in high-grade glioma patients using neuronavigation-guided focused ultrasound. npj Precis. Onc. 7, 92 (2023).
- First clinical trial of SDT in recGBM. 12 patients recruited in an ‘energy dose escalation’ trial with three cohorts (200J, 400J and 800J). Half of the patients’ tumour was sonicated 5-7 days prior to complete tumour removal and histological analysis to look for pathological evidence of cell kill. The trial showed that SDT was safe, with no Adverse Events (AEs), and histological analysis revealed that cell kill was achieved in the sonicated half of the tumour via reactive oxygen species release (i.e. the same mechanism of cell kill as photodynamic therapy). These safety and clinical proof of concept data have been submitted by Nader Sinai for publication in Science Translational Medicine but a preview is available on this video presentation by Stuart Marcus, recorded before the trial was completed: Stuart Marcus – Sonodynamic Therapy for DIPG (youtube.com)
Sanai N, Tien AC, Tovmasyan A, et al. CTNI-13. A first-in human phase 0/1 trial of 5-aminolevulinic acid sonodynamic therapy (5-ALA SDT) in recurrent glioblastoma. Neuro Oncol. 2022;24(suppl 7):vii72-vii73
Online Link
- First clinical trial results from use of an un-focused delivery device (CV-01 machine by Alpheus medical) that was used to deliver 3-4 SDT treatments one month apart to patients with recurrent high grade gliomas (NB: 2 of the 12 patients treated had recurrent Grade 3 oligodendroglioma, and 10 patients had recGBM): https://clinicaltrials.gov/study/NCT05362409
Although a safety trial, median survival of the treated patients was 15 months (as opposed to 6-8 months in historical controls). This is a greater improvement in overall survival than seen with tumour vaccines (e.g. DC-Vax), Tumour Treating Fields (Optune) or Bevacizumab (Avastin) – but note that these latter three experimental treatments have their survival benefit of 3-4 months calculated from adequately powered Phase 3 large clinical trials. Note that the CV-01 machine uses unfocused ultrasound and will therefore deliver less energy to deeper tumours than the Cordance Cap, which can deliver more energy deeply in brain (current theories suggest that higher energy delivery promotes greater cell kill)
Schulder M, Johns T, Mechtler L et al. Results from a phase I study of sonodynamic therapy with holohemispheric low-intensity non-ablative ultrasound in patients with recurrent high grade glioma. J.Neuro-onc (suppl 8): viii99.
- Safety publication from the first-in-human clinical trial of SDT for children with Diffuse Intrinsic Pontine Glioma (DIPG), also known as Diffuse Midline Glioma (DMG). This demonstrated no AEs or SAEs, with stabilisation of tumour growth. Subsequent unpublished presentations of this trial demonstrated reversal of neurological deficits and increase in median survival after Radiotherapy from 9 months to 19 months. This is unprecedented for DIPG / DMG, especially considering the trial was halted early and not all patients had all twelve of their planned monthly SDT treatments. Video of reversal of neurology courtesy of trial sponsor, SonALAsense, USA:
Syed HR, Kilburn L, Fonseca A, et al. First-in-human sonodynamic therapy with ALA for pediatric diffuse intrinsic pontine glioma: a phase 1/2 study using low-intensity focused ultrasound: technical communication. J Neurooncol. 2023; 162(2):449-451. https://clinicaltrials.gov/study/NCT05123534
- Review of Sonodynamic Therapy for Neurosurgeons with some preliminary data from SonALAsense’s Phase 1/2 study of SDT for GBM in the Mayo, USA before the trial was terminated due to a lack of funds. No AEs or SAEs were noted in this trial, which was not powered to look at survival, nor had the repeat treatments from (4 and 5) above which demonstrated a doubling of survival time for recGBM and DIPG respectively. NB: The trial has re-opened for recruitment with repeat SDT treatments in August 2025 (see ref.10 vi below)
Hutton D, Burns T. and Hossain-Ibrahim K. Sonodynamic therapy for recurrent glioblastoma. Preliminary data from a Phase 2 energy dose escalation trial. Neurosurgery Focus (Sept 2024) 57(3), E7.
- The first trial in Europe of SDT for recGBM to publish preliminary data, again showing no AEs or SAEs. (Note that the lead author has previously published data on the efficacy of PDT as a mechanism to treat GBM in a clinical trial setting – see Section 2.2 of reference 1 above – which we believe to be the same mechanism by which SDT works). This trial used monthly unfocused ultrasound treatments using the CV-01 machine:
Stummer W, Gerwing M, Bilgin SS, et al. Sonodynamic therapy with a single neoadjuvant, diffuse delivery of low-intensity ultrasound with 5-ALA in treatment naïve glioblastoma results in tumor-specific cytotoxic edema and increased apoptosis. J Neurooncol. 2025 May;172(3):687-693.
- The last trial to show a significant survival benefit for patients with GBM was in 2005 and the survival data within is often used as a comparator for ‘historic controls’. As a result of this trial radiotherapy and adjuvant temozolomide chemotherapy is now standard of care for GBM, after maximal safe surgical debulking / resection (the Stupp Protocol):
Stupp, R. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med (2005) 352, 987-996, doi:10.1056/NEJMoa043330
- Review paper discussing the possibility that SDT can have an abscopal effect – i.e. the antigen release from the cell kill causes an ‘auto-vaccination’ effect, as has been seen in PDT, and greater survival benefit than can be explained by the sonication field itself:
Collins VG, Hutton D, Hossain-Ibrahim K and Banerjee S. The abscopal effects of sonodynamic therapy in cancer. Br J Cancer (2025) 132: 409-420
10. Other clinical trials of SDT for glioblastoma that have either not yet been published or are still recruiting:
i. SonicALA clinical trial of SDT for newly diagnosed GBM prior to resection (safety study, Milan, Italy). This trial used the more invasive Insightec Exablate machine that requires skull pins to place a fixed helmet on the patient, with treatment performed lying still inside an MRI scanner (i.e. requiring considerably more resources, time and cost compared to the Cordance Cap – see comparator table below); https://www.clinicaltrials.gov/study/NCT04845919
ii. Continuation of Nader Sanai’s safety study (ref 2 above) to expand to 45 patients to gain preliminary efficacy data in patients with recGBM: https://clinicaltrials.gov/study/NCT04559685
iii. The first safety and feasibility study to investigate the use of the NaviFUS machine to deliver SDT to patients with recGBM (see comparator table below): https://clinicaltrials.gov/study/NCT06039709
iv. Trial of unfocused ultrasound with CV-01 device 2-5 days prior to surgical resection of GBM to evaluate safety and early histological effects (preliminary data published in reference 7 above): https://clinicaltrials.gov/study/NCT06665724
v. Phase 2 study of Alpheus Medical’s Low Intensity Diffuse (unfocused) Ultrasound for recurrent high grade gliomas (using the CV-01 machine – see comparator table below): Not yet registered with clinicaltrials.gov
vi. Phase 1b trial to test safety, best dose and preliminary efficacy data (n=8) of intravenous ALA with the Insightec Exablate MR-guided FUS device. Treatments are to be repeated every 6 weeks until disease progression or unacceptable toxicity: https://clinicaltrials.gov/study/NCT07076472
vii. First Randomised 1:1 trial to compare SDT + Standard of Care to SOC alone (radiotherapy, temozolomide and bevacizumab) for patients with newly diagnosed GBM, in Zenghzhou, China (2025). Aiming to recruit 220 patients, using intra-arterial Hiporfin® as the sonosensitiser (which requires avoidance of sunlight for 30 days) and treatments every day for 3 days followed by 24 mths monitoring. Note Phase 1 study not published: https://clinicaltrials.gov/study/NCT07130149
viii. First Randomised 1:1 trial to compare SDT + Standard of Care to SOC alone (radiotherapy, temozolomide and bevacizumab) for patients with DIPG, in Zenghzhou, China (2025). Aiming to recruit 216 patients using intravenous Hematoporphyrin (‘Xipofen’) twice per day for 4 days. Note that their Phase 1 study showed only a two month survival benefit and there is little data published on their handheld ultrasound device: https://clinicaltrials.gov/study/NCT06999148
Competition in Development
Comparison of Cordance-Cap SDT to Chemotherapy, Radiotherapy, and Optune (Tumour Treating Fields), which demonstrated 3-4 month extra survival in GBM.